Opportunities
Currently seeking applicants/volunteers for the following positions:
- 2 High School Teachers for a 6-week long paid Summer Research Experience for Teachers (SRET) Program
- Undergraduate Volunteers for Gene Therapy Projects
- Undergraduate Volunteers for Blood Substitute Projects
Summer 2019 Research Experience for (High School Science/Biology/Chemistry) Teachers (SRET) Program
Currently looking for 2 high school teachers of science, biology, or chemistry to participate in a Summer Research Experience for Teacher (SRET) program that is sponsored by the National Science Foundation. Participants will be given a stipend of $5,000 to spend 6 weeks in my lab over the summer of 2019 conducting cutting edge experiments that contribute to one of our ongoing gene therapy research projects. After the conclusion of the SRET, I will also work with each participant to develop a lesson plan in which the teacher will share their SRET experience with their students in the following academic year. The lesson will include a description of the experiment(s), analysis of data, and videos of the teacher performing actual experiments to personalize the lesson. After that lesson, a short survey will also be given to the students to assess the impact of the lesson on their career goals.
Any interested applicants should submit questions and/or their resume to Dr. Elmer (jacob.elmer@villanova.edu) by April 1st, 2019.
Gene Therapy Research Opportunities for Undergraduates
Overview: Animal cells are very good at detecting early signs of pathogenic infection. For example, when foreign DNA enters the cytoplasm, it triggers DNA sensor proteins that activate a signaling cascade of kinases and transcription factors that ultimately induce the expression of inflammatory cytokines and other genes that help defend the cell and/or organism from the pathogen. Unfortunately, while this “innate immune response (IIR)” helps us to effectively repel viruses and bacteria, it can also significantly hinder gene therapy. Indeed, gene therapy techniques also introduce DNA into the cytoplasm, which then triggers the IIR and leads to transgene silencing. The purpose of the projects described below is to improve gene therapy by manipulating the IIR in different ways to increase transgene expression.
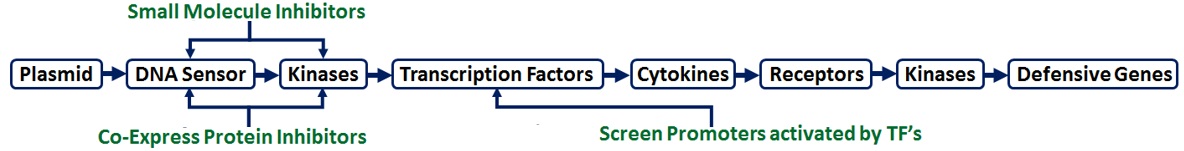
Inhibition of the IIR with Small Molecule Inhibitor Drugs
Although the diagram shown above appears quite simple, it actually involves dozens of different DNA sensors, kinases, and transcription factors. We have previously shown that inhibiting some of those proteins with small molecule inhibitor drugs can significantly increase transgene expression (e.g. up to 30-fold with iCRT14, an inhibitor of the transcription factor b-catenin). Each student involved in this project will be responsible for testing the effects of additional inhibitors (e.g. quinacrine, ML60218, NU7026, NU7441, Bay11-7082, IKK16, 2-aminopurine, etc.) on transgene expression, cell viability, and inflammatory cytokine expression (e.g. IL-6).
Inhibition of the IIR via Co-Expression of Anti-Inflammatory Proteins
As an alternative to small molecule inhibitor drugs, some viruses and even some human cell types express proteins that inhibit the IIR. For example, IL-2, IL-10, and IL-37 are anti-inflammatory cytokines, while SOCS1 is an intracellular protein that interferes with JAK/STAT signaling. Students involved in this project will utilize these inhibitory proteins in 2 ways: (1) co-expression of the transgene with the anti-inflammatory protein(s) inside the host cell or (2) expression of recombinant proteins in E. coli, which can then be added to the host cell during transfection. The effects of the proteins on transgene/cytokine expression and cell viability will then be measured.
Hijacking the IIR with Cytokine-Inducible Promoters
The IIR is a highly redundant system, such that inhibition of a single component of it may not be sufficient to prevent transgene silencing. In this case, the motto is, “If you can’t beat ‘em, join em.” Specifically, students involved in this project will drive transgene expression with 6 different promoters that are known to be activated in the IIR (IFNa1, IFNb1, IFNg, IL6, IL8, TNFa), thereby allowing us to effectively hijack the IIR. The strength of each promoter will be determined by measuring transgene expression and mRNA levels inside the cell with rt2PCR.
Development of Earthworm Hemoglobin as a Blood Substitute – Opportunities for Undergraduate Research

Creation of Ultra-Stable Blood Substitutes via Polymer Conjugation
We have already shown that conjugating the polymer polyacrylic acid (PAA) to LtEc significantly increases its thermal stability. Students involved in this project will take the next steps by conjugating other polymers to LtEc: Higher/lower MW PAA, Polysialic acid (PSA), polyethylene glycol (PEG), and alginic acid. In addition, one student may also be responsible for attaching the fluorophore DY-676 to LtEc so we can track its fate in vivo. In each case, the effects of conjugation on thermal stability, iron oxidation, and size will be determined. The modified Ecs with the most favorable properties will also be transfused into mice.
Freeze-Dried Earthworm Hemoglobin as an Ultra-Portable Blood Substitute
The goal of this project is to decrease the weight of a unit of LtEc to make it Ultra-Portable. Specifically, LtEc will be frozen and then dried under vacuum to obtain a protein powder, which can subsequently be dissolved in water. Freeze drying parameters will be optimized, while the effects of this process on LtEc stability and heme iron oxidation will be measured.
Improving Animal Cell Culture via Earthworm Hemoglobin O2 Delivery
Since hemoglobins support cell growth in vivo by delivering oxygen to tissues, it is possible that they can also enhance cell growth in vitro as well. The goal of this project will be to determine the effects of supplementing human cancer cells (prostate, breast, and T-cell types) with LtEc. Students will measure cell growth rates and viability while tracking LtEc oxidation/aggregation.
Note: These projects are part of a National Institutes of Health (NIH)-sponsored program that involves a direct collaboration with the Children’s Hospital of Philadelphia. Students participating in these projects will go to CHOP at least 3-4 times to observe animal experiments, learn new techniques, and attend/participate in lab meetings.
