Gene Therapy Research
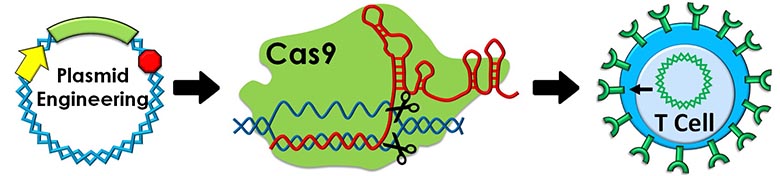
Gene therapy has the potential to cure many different genetic disorders, but our research focuses on using gene therapy to treat cancer (e.g. leukemia and prostate cancer). Specifically, our main goal is to optimize the non-viral delivery, expression, and genomic integration of specific genes (e.g. CAR, Cas9, GFP, etc.) that reprogram T cells to seek out and attack cancer cells. We are currently investigating several methods to enhance the expression of foreign genes within T cells, including:
Epigenetic Control of Transgene Expression
Image: Nucleosome, PDB ID 1AOI
The viral and bacterial DNA used in gene therapy is often condensed by histone proteins and sequestered into tightly packed heterochromatin after delivery to the nucleus, which ultimately leads to silencing of the delivered transgene(s). Our goal is to prevent transgene silencing by inserting “enhancer” sequences into our plasmids that cause them to take on a more relaxed/open conformation (i.e. euchromatin) instead of heterochromatin.
Manipulation of the Innate Immune Response
Image: NF-kb bound to DNA, PDB ID 1SVC
When foreign bacterial or viral DNA enters a cell, it can trigger a variety of defense mechanisms that include inhibition of translation/protein expression, transgene silencing, and/or apoptosis (cell death). This innate immune response (IIR) presents a significant problem for most gene therapy treatments, since they utilize bacterial or viral DNA. We are currently addressing this problem by (1) identifying the specific enzymes/proteins involved in the IIR, (2) inhibiting key parts of the IIR, and (3) hijacking proteins that are activated during the IIR and using them to boost transgene expression.
Optimization of Cas9 Expression/Activity in T Cells
Image: Cas9, PDB ID 4CMP
CRISPR/Cas9 technology has enabled researchers to easily mutate genes, but inserting a gene into the genome with Cas9 is still a challenging task (especially in T cells). We aim to optimize every step in Cas9-mediated genome editing in T cells, from gene delivery to genomic insertion via homology directed repair, all with the ultimate goal of maximizing chimeric antigen receptor (CAR) expression in primary T cells.
Grants

We sincerely thank the National Science Foundation for their continuing support of our research!
NSF 1403214:BBBE:Manipulating Epigenetic Mechanisms to Enhance Non-Viral Transgene Expression. 2014-2017, $600,000 (a collaboration with Arizona State; $174,000 for Dr. Elmer as Co-PI)
NSF 1645225:CBET:EAGER:
NSF 1651837:CBET:CAREER:
Publications
Harris E, Zimmerman D, Warga E, Bamezai A, Elmer J. Non-Viral Gene Delivery to T Cells with Lipofectamine LTX. Biotechnology & BioEngineering. 2021. DOI: 10.1002/bit.27686
Spivack K, Muzzelo C, Hall M, Warga E, Neely C, Slepian H, Cunningham A, Tucker M, Elmer J. Enhancement of Transgene Expression by the b-catenin Inhibitor iCRT14. Plasmid. 2021. 114: 102556.
Harris E, Elmer JJ. Optimization of electroporation and other non-viral gene delivery strategies for T cells. Biotechnology Progress. 2020. e3066. https://doi.org/10.1002/btpr.3066
Barret CM, McCracken R, Elmer J, Haynes KA. Components from the human c-myb transcriptional regulation system reactivate epigenetically repressed transgenes. Int. J. Mol. Sci. 2020 21: 530.
Elmer J, Christensen MD, Barua S, Lehrman J, Haynes KA, Rege K.
Christensen MD, Elmer J, Eaton S, Gonzalez-Malerva L, LaBaer J, Rege K.
Elmer J, Christensen MD, Rege K. Applying horizontal gene transfer phenomena to enhance non-viral gene therapy. J Control Release. 2013 172(1):246-257.

